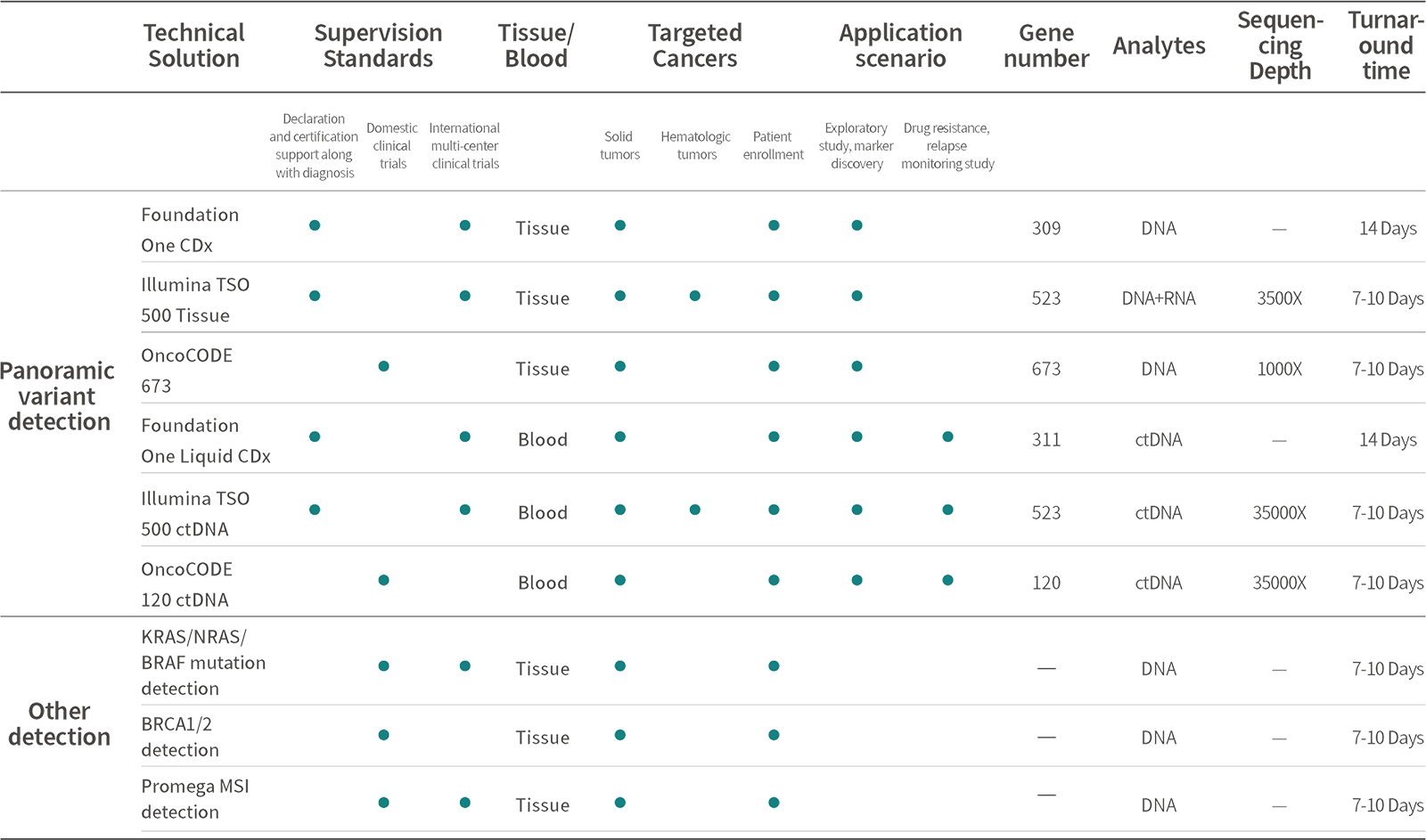
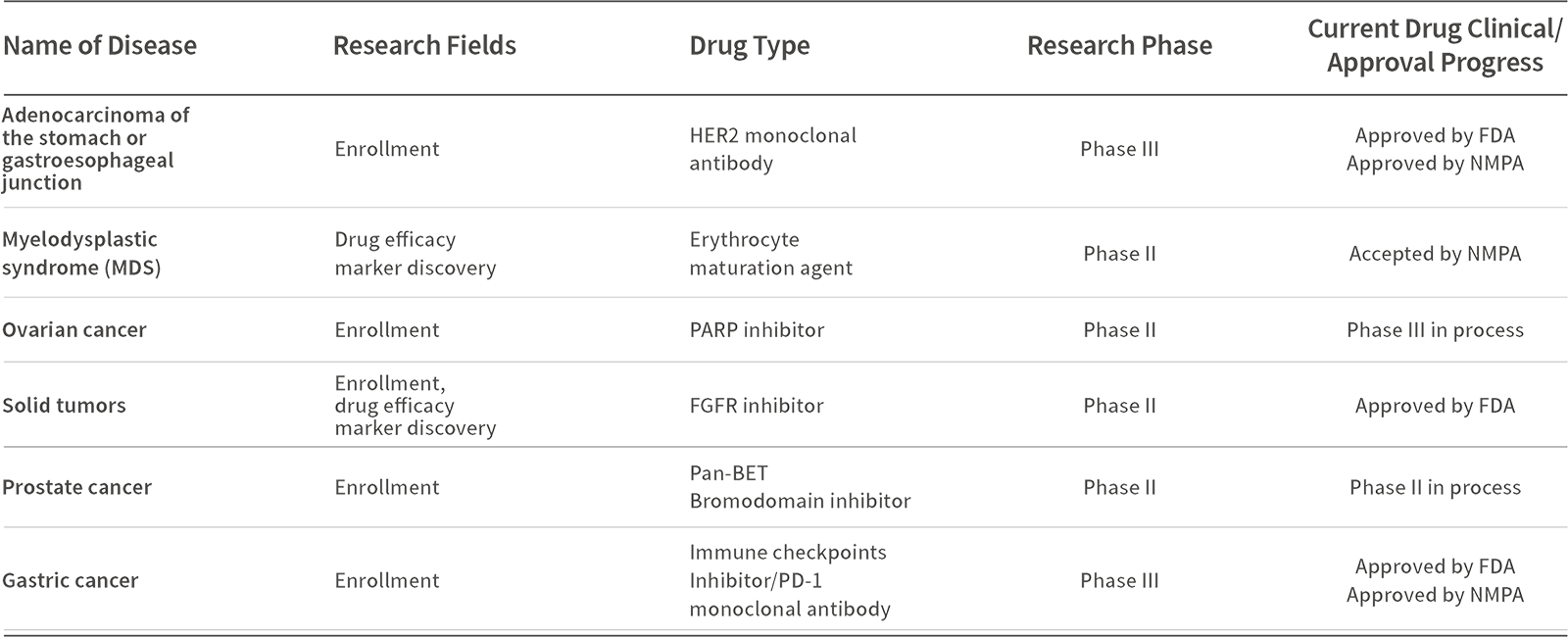
Pan-cancer comprehensive genomic profiling products of Sequanta Technologies have been validated by Sequanta Laboratories to meet CAP/CLIA standards, including accuracy studies, precision studies, and limit of detection studies, and have passed CAP, NCCL, SCCL, and other domestic and international inter-laboratory quality assessments with perfect scores, thus fully meeting the performance requirements of regulatory agencies for test in clinical trials .